OV350 & KCC2 platform
overview
Ovid’s K+Cl– cotransporter 2 (KCC2) program is a portfolio of potential first-in-class direct activators of the KCC2 transporter. These activators may have therapeutic application in rare and common epilepsies, as well as other CNS indications. Ovid exclusively in-licensed the KCC2 portfolio from AstraZeneca in 2022.
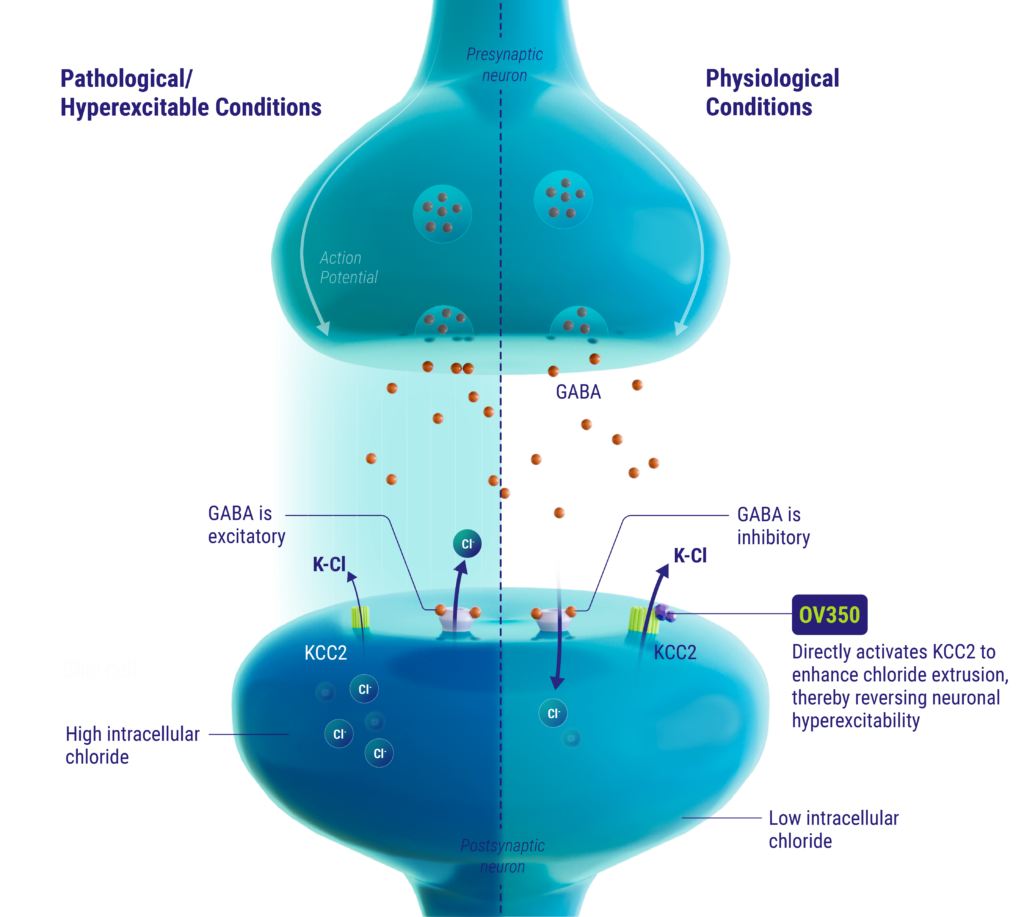
mechanism of action
The KCC2 transporter is a neuron-specific chloride extruder that is critical for maintaining GABA’s inhibitory function in the mature brain. Deficits in KCC2 activity have been associated with epilepsy and other neuropathological disorders. OV350 directly activates KCC2, thereby restoring chloride homeostasis in neurons and subsequently reducing hyperexcitability.
development
In vivo proof-of-concept studies in animals have established that restoring KCC2 activity leads to reduced seizure sensitivity and seizure-induced mortality. In one preclinical model, designed to mimic the acute seizure state of status epilepticus (SE), OV350 in combination with diazepam terminated seizures, restored the efficacy of diazepam, and reduced the amount of associated neuronal loss following injury. Preclinical mechanistic studies have also demonstrated that OV350 was well-tolerated and did not induce sedation.
In 2022, Ovid evaluated several compounds in the KCC2 portfolio and began optimizing the lead candidate, OV350, for multiple possible formulations. Our desire is to achieve both intravenous and oral administration formulations for OV350. Dual formulations are optimal for patients who are treated acutely in the hospital and need to maintain seizure reduction in an out-patient setting.
For additional information on OV350 non-clinical data, please click here.
potential indications
Ovid is analyzing multiple candidates from the KCC2 portfolio for development in epilepsy as well as other possible neurological conditions.